Technology
ImotopesTM: Next Generation
Immunotherapeutics
ImotopesTM are a next generation immunotherapy that have the potential to address a wide range of diseases in the field of immunology. Our current focus is to find new therapies for patients suffering from severe, chronic autoimmune diseases where our pioneering approach could prevent, treat and potentially cure some of the most challenging and underserved conditions.
The ImotopeTM Technology
The challenge of current therapies
Autoimmune diseases occur when the body’s immune system mistakenly attacks the body’s cells and tissues. Few therapies address the underlying causes of the disease and those that do often cause a generalized immune suppression, to the point that the immune system cannot mount a proper response leading to severe side effects such as the inability to fight infection or control cancers. The side effects from some treatments are so severe that many patients stop treatment even if they are somewhat effective.
Existing therapies can also require daily medication or periodic hospital infusions.
Most other drugs simply alleviate the symptoms or temporarily halt the immune attack.
For all autoimmune disorders there are no cures and innovation is needed.
Imotopes™ are simple peptide molecules, injected sub cutaneously, which stimulate an immune response that blocks the autoimmune pathways. They have the potential to have long lasting effects that stop the progression of the disease.
Current therapies are generally:
ImotopesTM – a new solution
Autoantigens are natural proteins that are immunogenic when normal tolerance is broken (where the body’s immune system sees them as “foreign” and begins to mount an immune response).
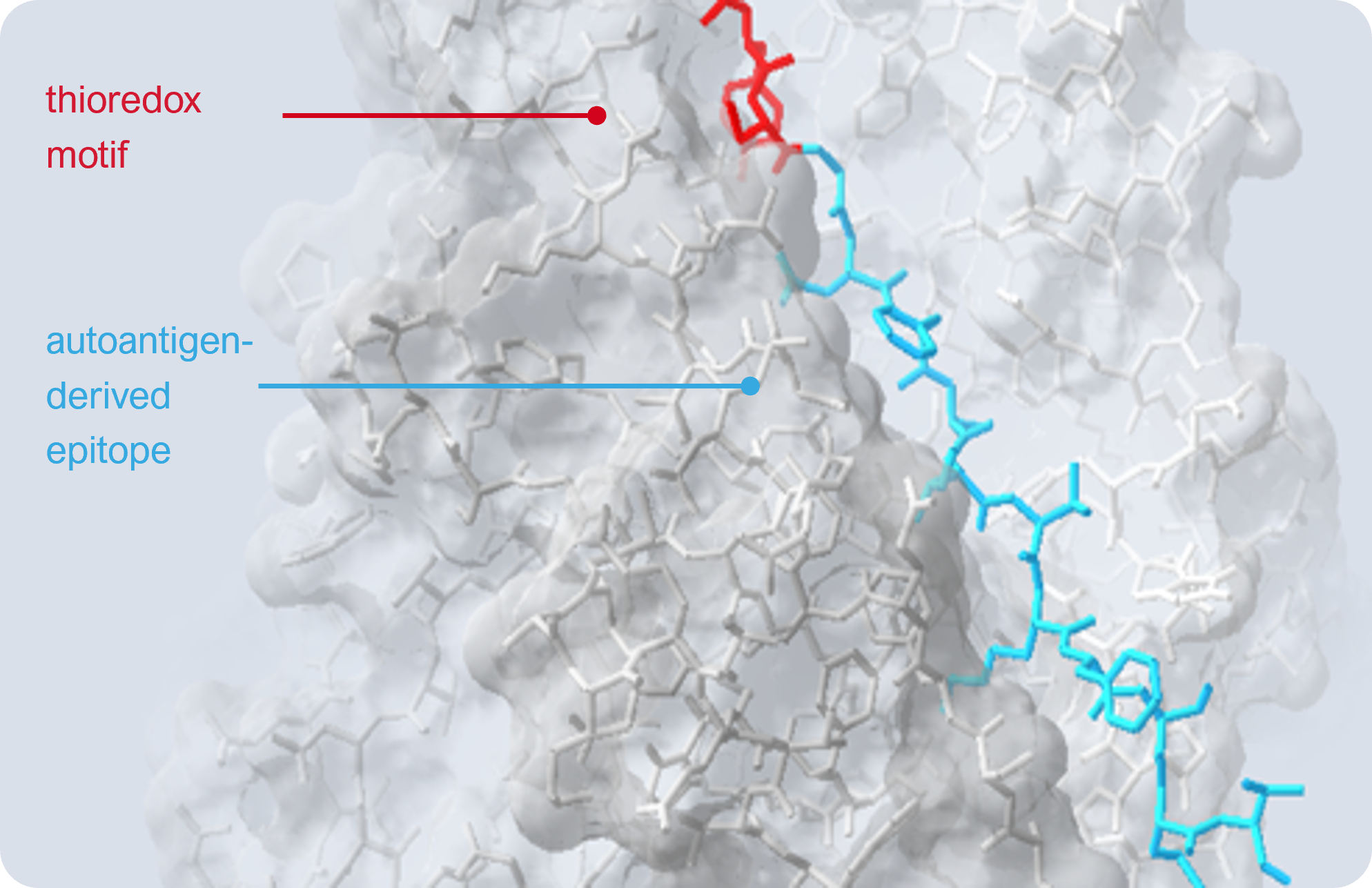
Imotopes™ are short peptides that simulate an immune response to rebalance the aberrant autoimmune response. We take a sequence from the autoantigen called epitope (the light blue) and construct our peptide from that core. Importantly we add a sequence from the thioredoxin enzyme (the red) to give this peptide its biological effect.
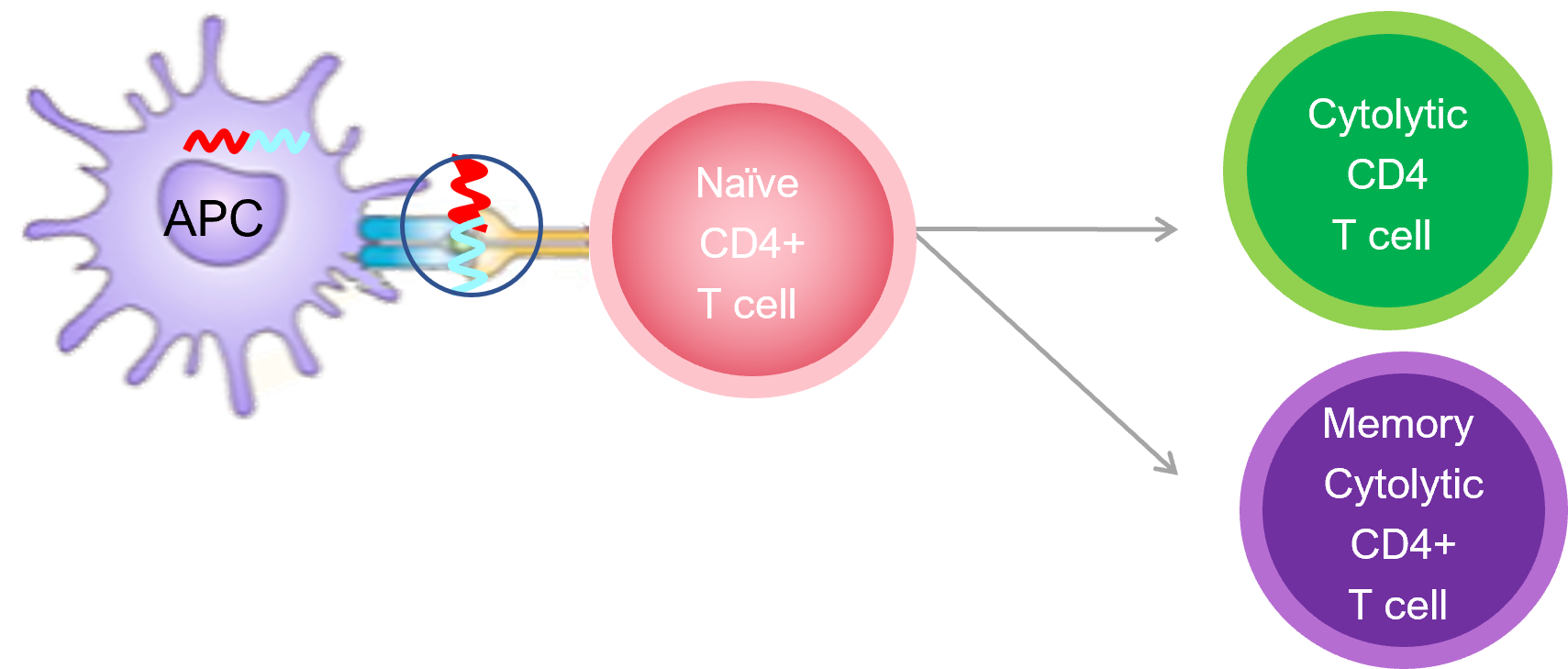
When ImotopesTM are injected, they stimulate a new class of immune cells, called cytolytic CD4+ cells that specifically targets the disease pathway and knocks out a key cells in the autoimmune cascade. Importantly, these cytolytic T cells do not affect the rest of the immune system which continues to function. By interrupting the autoimmune cascade, Imotopes™ prevents further tissue damage and halts disease progression.
Imotopes™ also induce memory cytolytic CD4+ T cells, which provide long-lasting treatment effects with infrequent dosing, like a vaccine.
Broad applicability
In addition to autoimmune diseases, Imcyse’s technology has potential in allergy, cancer and other diseases.
The technology could also be applied as an “add-on” therapy to other drugs or biologics to prevent the progression of immunogenicity and loss of efficacy with chronic administration - such as with monoclonal antibodies or replacement enzymes. Furthermore, it may eliminate the immune response against the viral vectors that are used in gene therapies.
A closer look at the development of autoimmune diseases and how ImotopesTM tackle the key players in the immune cascade for a specific, safe and long lasting therapy.
The underlying immune mechanisms in the development of autoimmune diseases
Autoimmune disorders (AIDs) occur when the immune system mounts an attack to self-proteins and initiates an immune cascade that ultimately attacks the body’s own tissues and organs.
In healthy individuals, unwanted immune responses to self-proteins are kept in check, which is called tolerance. When tolerance is broken for a specific self-protein, it is able to stimulate an immune response and, are then called autoantigen. Like all proteins, these autoantigens are routinely taken up by antigen-presenting cells (APCs) but only when tolerance is broken, they ultimately activate disease causing autoreactive T cells, also called pathogenic T cells, a key first step in the inception of autoimmune disease.
On the surface of APCs MHCII molecules present protein fragments, also called epitopes, to T cells. A given autoantigen has many different epitopes which are presented on MHCII molecules. The MHCII molecules and the genes encoding them are highly variable between individuals and are the strongest genetic risk factor to develop a certain autoimmune disease, demonstrating the critical role of these MHCII molecules and the epitopes they present to T-cells in the development of autoimmune diseases. For example, Type-1 diabetes is associated with MHCII molecules DR3 and DR4 that can present preproinsulin-derived epitopes reported to play a role in this autoimmune disease.
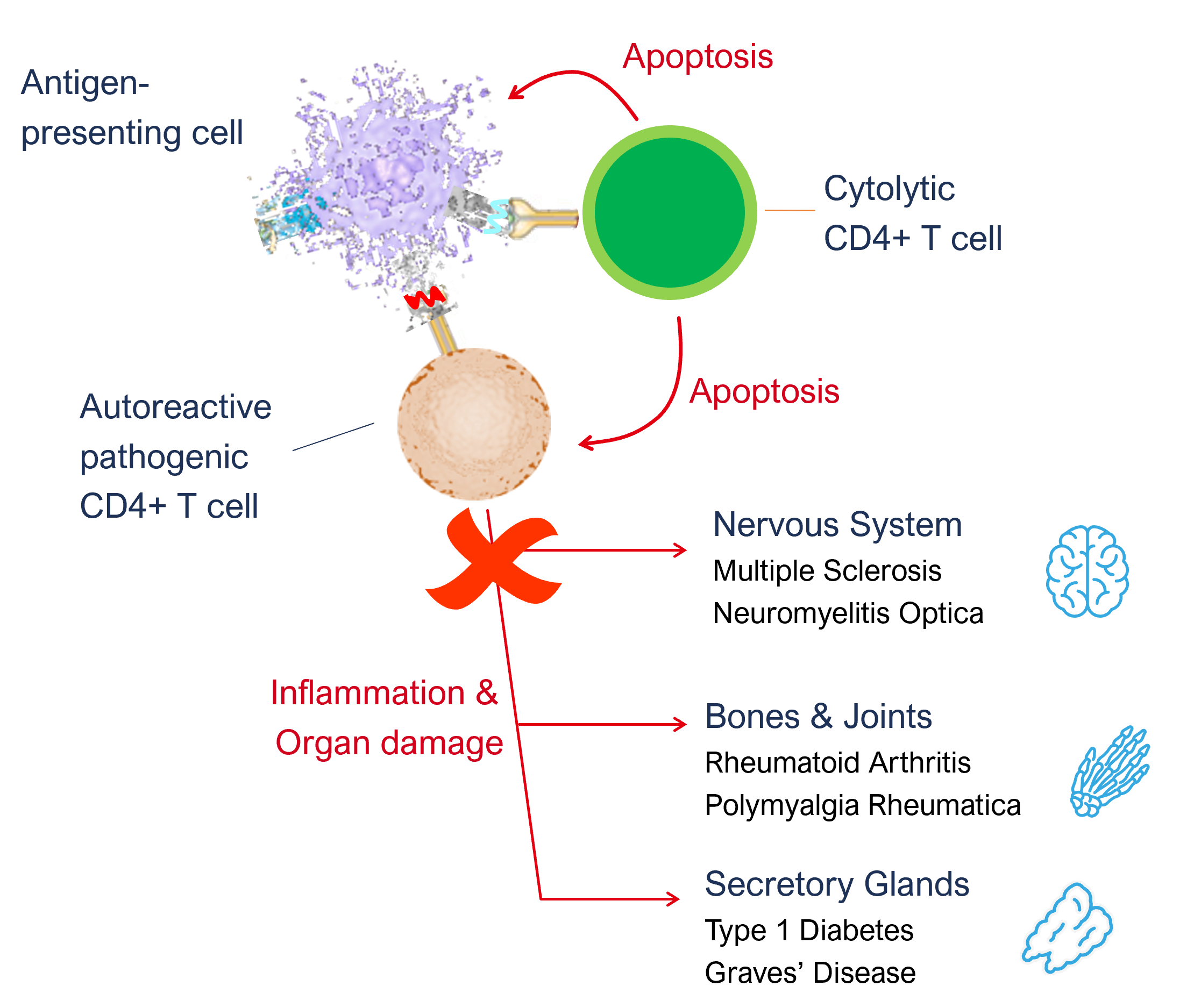
APCs begin the disease cascade by processing and presenting autoantigen epitopes to T cells. The peptide-MHCII complex binds with the T cell receptor (TCR) on the CD4+ T cell and forms the immune synapse. In patients with autoimmune conditions this presentation of the body’s own protein epitopes stimulate the T cell and causes it to proliferate and differentiate into a pathogenic T cell phenotype. These pathogenic T cells in turn stimulate different pathways inducing inflammation and damage through a variety of mechanisms (using antibodies/ B cells and CD8+ T cells as well as CD4+ T cell pathways).
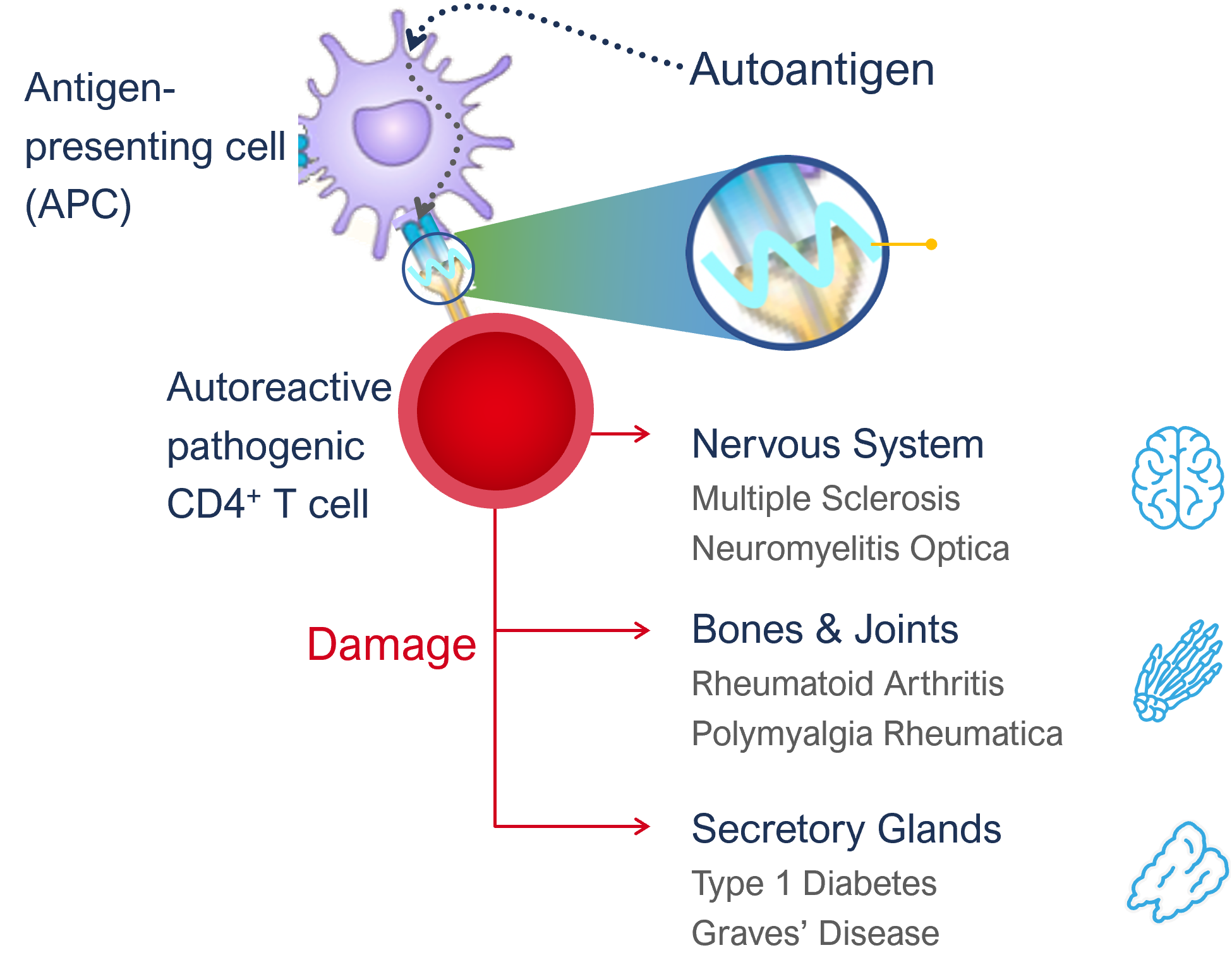
Antigen presentation by APCs is not only critical in generating pathogenic cells in the first place, it is also key in restimulating existing pathogenic T cells further driving/progressing the pathology (extends their life span). Without constant antigen presentation, T cells will simply wane in their activity and fade into a death spiral.
ImotopeTM technology: tackling the key players in the immune cascade by re-deploying a natural response to treat autoimmune diseases
Imcyse’s Imotope™ approach aims to break this aberrant immune cascade and reset the immune system by using these disease causing epitopes. This is made possible through the Imotope’s™ unique design which targets specific MHCII haplotypes.
The first step in designing the ImotopeTM is to examine the proteins that have become autoantigens and the epitopes that cause the disease. Once the epitope that we wish to use has been identified, it is then modified by adding a sequence from an enzyme called Thioredoxin (which we call a “thioredox” sequence or motif). The addition of the thioredox motif gives the peptide, now called an ImotopeTM, the power to drive T cells into a different phenotype, called a cytolytic CD4+ T cell. Cytolytic CD4+ T cells have previously been reported during viral infections and are shown to kill virus-infected APCs. We are re-deploying this mechanisms for the treatment of AIDs.
As the Imotope™ has the autoantigen’s epitope at its core, the cytolytic T cells that are generated are epitope specific. For example, we chose an epitope from pro-insulin as the core of our Type 1 diabetes Imotope™ which when injected generates insulin specific cytolytic T cells. This makes the response to the treatment very targeted and allows for a treatment that is both safe and effective.
Once the body has primed a new army of disease autoantigen epitope specific cytolytic CD4+ T cells, they circulate around the body. If they encounter another APC presenting anything other than the autoantigen epitope from which they were primed, no T cell activation occurs. However, when these cells encounter an APC presenting the autoantigen epitope, they get activated and induce apoptosis in that APC. This removes a critical step in the disease pathway and prevents the body from generating new pathogenic T cells, effectively stopping the disease. By only inducing apoptosis in APCs that are involved in the disease pathway, our treatment does not have a negative impact on the general immune system.
Not only does an Imotope™ induce a cytolytic CD4+ T cell soon after treatment, it also induces a memory phenotype, like vaccines do. Memory cytolytic CD4+ T cells are long lived and are able to continue to counter the aberrant immune response for long periods of time. This means that an Imotope™ course of therapy will have a very long duration of action/efficacy, which has been observed in our pre-clinical experiments so far, and could even lead to disease remission or cure.
In addition to inducing apoptosis in the APC, we also observe that cytolytic CD4+ T cells can induce apoptosis of the pathogenic T cells, as they are interacting with the same APCs. This is a second and equally important component of the therapeutic efficacy of Imotopes™ as they eliminate pathogenic T cells further contributing to potential remission or cure.
Bystander Effect
Most autoimmune diseases have several self-proteins which have the potential to become immunogenic (autoantigens), sometimes increasing in number as the disease progresses and the immune dysregulation rages more and more out of control. As APCs at the site of tissue damage will be processing many of the proteins found in the local environment, we have confirmed it is likely that one APC will be presenting several autoantigens at any one time and the pathogenic T cells primed from a number of related autoantigens and epitopes will all be interacting with a single APC. We have observed that a cytolytic CD4+ T cell primed with one epitope can in these circumstances induce apoptosis in related pathogenic T cells which enables the Imotope™ to block a wider range pathogenic T cells, maximizing the therapeutic benefit.
In summary, the ImotopesTM are able to block the abnormal immune responses and recalibrate the immune system, breaking the disease pathway responsible for tissue damage. ImotopeTM-induced cytolytic T cells specifically eliminate antigen-presenting cells and pathogenic T cells, thereby avoiding autoimmune attacks without affecting other functions of the immune system.
Further resources
-
Publications
-
Posters
-
Additional sources